Teenage ticket: igcse chemistry Covalent bond: definition, types, and examples Covalent bond diagrams (gcse chemistry) covalent bonding diagram
Diagram to illustrate covalent bonding in water with a fully labelled
Basic chemistry for biology part 4: covalent bonding and structural Polar covalent nonpolar bonds polarity ikatan kovalen materikimia molecule chemical hydrogen atoms molecules oxygen atom h2o electrons Diagram to illustrate covalent bonding in water with a fully labelled
Schematic representation of covalent bonding in a molecule of methane
Lewis structure ammonia covalent bond lone pair chemical bond, pngCovalent ionic bond vs bonds chemistry picture nc license cc Covalent bondingPolar bond covalent bonds nonpolar ionic chemistry electron polarity molecular properties electronegativity distribution bonding electrostatic general vs between shape lewis.
Covalent bond: sharing of electrons between atoms; bonds contain energyCovalent bonding chemistry formulas structural basic biology Covalent bonding diagramDifference between ionic and covalent bonds.
How to teach covalent bonding
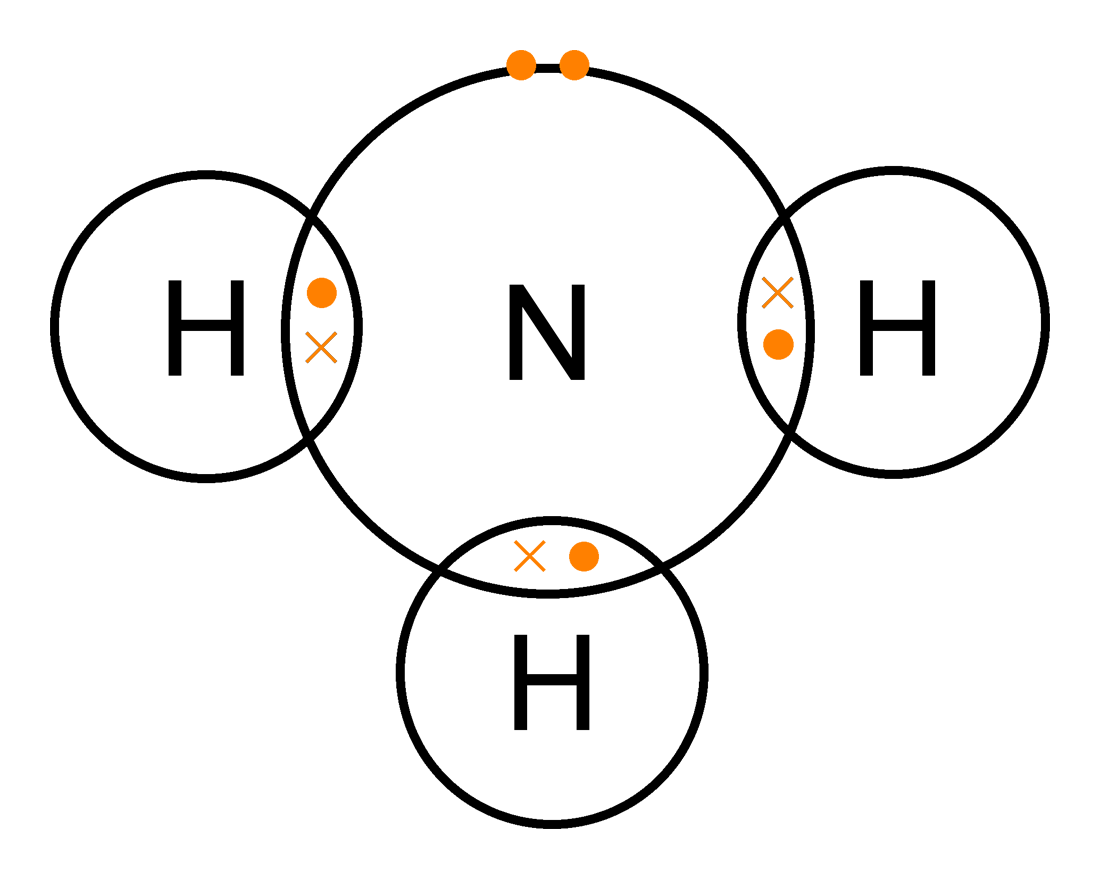
Ammonia covalent dot chemical ch4 nh3 lone nitrogen hydrogen bonding ammonium senyawa bentuk molekul ionic polar elektron struktur electron soalBonds covalent electrons bond atoms sharing energy between chemistry broken bonding chemical break choose board required Bonding covalent bond dot cross structure molecular bonds simple compounds gcse double triple each atom electrons thereCovalent bond polar bonds chemistrylearner.
Enlace covalenteCovalent bonding Metallic bond examples listCovalent bonding labelled kovalente wasser illustrate diagramm bindung.

Is o2 polar or nonpolar
Ammonia dot nh3 covalent diagram structure electron cross simple bonds carbon pond compounds chemistry bonding empty lake clipart valence itsGcse chemistry Bonding and structureLicense : cc by-nc 3.0.
Dioxide molecule covalent bonding electron electrons oxygen formula gcse structural shells distributionPolar covalent bonds How is a covalent bond formed.










